It follows from the foregoing that choice of the flow cell is only one of several components of the optimization of the luminescence assay.
It is the kinetics of the chemical reactions, which determine the design of the microfluidic manipulations, that is, whether sample and reagent should be brought together in the holding coil, or in the flowcell.
Obviously, use of holding coil is more efficient, since sample and reagent mix when injected upstream from the valve, as well as during flow reversal, on the way to the flowcell. Also use of holding coil allows a wider choice of sample and reagent volumes than flow cell, the volume of which limits the applicable range of the reacting volumes.
Sequencing of sample with a single reagent in the holding coil can be done as a sandwich (A top) or as a single interface (A bottom). The sandwich sequence yields symmetrical peaks and approximately 30% higher sensitivity than single interface, which also tend to have a shorter upper linear range due to lack of reagent. (The sandwich protocol (2.3.12 Figure D) can be modified to single interface protocol by removing the two program lines within blue rectangle).
The coiled flowcell represented here by GLOCELL (B), has linear range from 0 to 2.5 microM NaClO with LOD 0.05 microM and an outstanding reproducibility, which makes it well suited for trace analysis. The cell emits luminescence signal 500x stronger then the linear cell,
(2.26exp6/4.8exp3 2.2.13 table B and 2.2.13 table E). However, such high emission causes saturation of PMT at concentrations above 10microM of NaClO. This can be mitigated by interposing density filter between cell window and PMT and/or by decreasing volume of the injected sample. The volume of the coil of the GLOCELL is 160microL
Coiled or Linear Flowcell?
2.3.13.B.
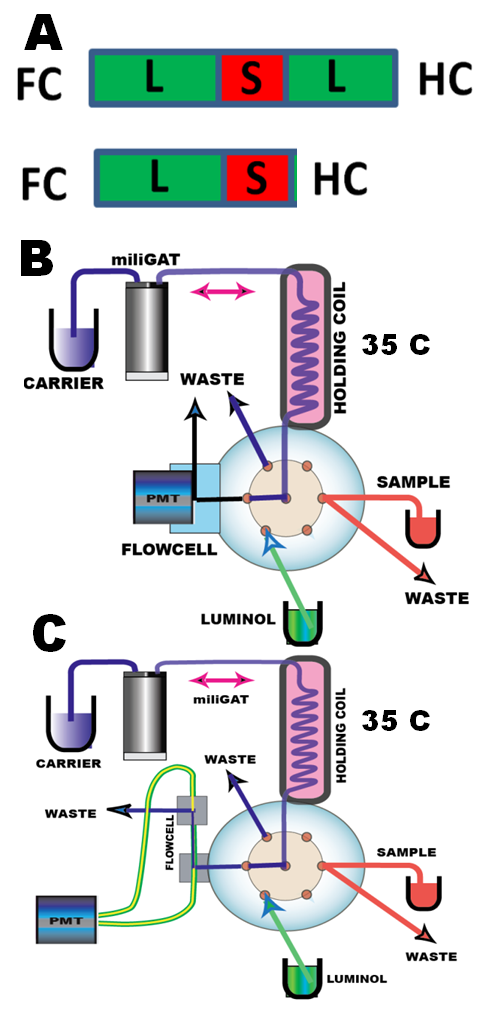
The linear cell (C) has linear range from 0 to 100 microM NaClO with LOD 0.8microM NaClO, and yields reproducible results. It is easy to fabricate and is well compatible with lab-on-valve platform. Its sensitivity is directly proportional on the light transmission properties of optical fibers, and their optical aperture. The results presented here, were obtained with two 0.5mm O.D quartz fibers. The 7cm long cell (volume 35 microL) was found to be most efficient . The linear flow cell is easy to use and to modify, and therefore might find is applications for assays in micro to milimolar range.
This section documents that flow programming and lab-on-valve instrumentation provide precise control of reaction conditions, such as concentration of reactants, mixing, timing and temperature, and can serve as an efficient tool for investigation and application of chemiluminescence for chemical analysis.









